

Amandla okucutheka kwawo nabuphi na ubude beyunithi kumphezulu wolwelo kuthiwa yingcinezelo yomphezulu, kwaye iyunithi yi-N.·m-1.

Ipropathi yokunciphisa ukuxinana komphezulu wesinyibilikisi ibizwa ngokuba ngumsebenzi womphezulu, kwaye into enale propati ibizwa ngokuba yinto esebenza phezu komhlaba.
I-substance-active substance enokuthi ibophe iamolekyu kwisisombululo esinamanzi kwaye yenze i-micelles kunye neminye imibutho, kwaye ibe nomsebenzi ophezulu ophezulu, ngelixa iphinda ibe nefuthe lokumanzisa, i-emulsifying, i-foaming, i-washing, njl.
I-Surfactant yi-organic compounds enesakhiwo esikhethekileyo kunye nepropathi, enokutshintsha ngokuphawulekayo ukunyanzeliswa kwe-interfacial phakathi kwezigaba ezibini okanye ukuxinwa komphezulu wolwelo (ngokubanzi kwamanzi), ngokumanzisa, ukukhupha amagwebu, i-emulsifying, ukuhlamba kunye nezinye iimpawu.
Ngokumalunga nesakhiwo, i-surfactants inento eqhelekileyo kuba iqulethe amaqela amabini endalo eyahlukeneyo kwiimolekyuli zabo.Kwesinye isiphelo kukho ikhonkco elide leqela elingekho kwi-polar, elinyibilikayo kwioli kwaye linganyibiliki emanzini, eyaziwa ngokuba yi-hydrophobic group okanye iqela eligxotha amanzi.Iqela elinjalo lokugxotha amanzi lihlala lihamba ngamatyathanga amade e-hydrocarbons, ngamanye amaxesha kunye ne-organic fluorine, i-silicon, i-organophosphate, i-organotin chain, njl.Iqela le-hydrophilic kufuneka libe ne-hydrophilic ngokwaneleyo ukuqinisekisa ukuba i-surfactants yonke i-soluble emanzini kwaye ine-solubility efunekayo.Ekubeni i-surfactants iqukethe amaqela e-hydrophilic kunye ne-hydrophobic, inokunyibilika ubuncinane kwisigaba esinye solwelo.Le propathi ye-hydrophilic kunye ne-lipophilic ye-surfactant ibizwa ngokuba yi-amphiphilicity.


I-Surfactant luhlobo lweeamphiphilic molekyuli ezinamaqela amabini e-hydrophobic kunye ne-hydrophilic.Amaqela e-Hydrophobic of surfactants ngokubanzi aqulunqwe ngee-hydrocarbons ezinde, ezifana ne-alkyl-chain-C8~C20, i-branched-chain alkyl C8~C20,alkylphenyl (inombolo ye-alkyl carbon tom yi-8~16) kunye nokunye okunjalo.Umahluko omncinci phakathi kwamaqela e-hydrophobic ubukhulu becala kutshintsho lwesakhiwo samatyathanga e-hydrocarbon.Kwaye iintlobo zamaqela e-hydrophilic zingaphezulu, ngoko ke iipropati ze-surfactants zihambelana ikakhulu namaqela e-hydrophilic ngaphezu kobukhulu kunye nokuma kwamaqela e-hydrophobic.Utshintsho lwezakhiwo zamaqela e-hydrophilic zikhulu kunezo zamaqela e-hydrophobic, ngoko ke ukuhlelwa kwee-surfactants ngokuqhelekileyo kusekelwe kwisakhiwo samaqela e-hydrophilic.Olu lwahlulo lusekwe ekubeni ngaba iqela le-hydrophilic yi-ionic okanye hayi, kwaye lahlulwe layi-anionic, i-cationic, i-nonionic, i-zwitterionic kunye nezinye iindidi ezikhethekileyo ze-surfactants.

① Ukufakwa kwee-surfactants kwi-interfac
Iimolekyuli ze-surfactant ziiamphiphilic molekyuli ezinamaqela amabini e-lipophilic kunye ne-hydrophilic.Xa i-surfactant inyibilika emanzini, iqela layo le-hydrophilic litsalwa emanzini kwaye linyibilika emanzini, ngelixa iqela layo le-lipophilic ligxothwa ngamanzi kwaye lishiya amanzi, okukhokelela ekufakweni kweemolekyuli ze-surfactant (okanye i-ion) kwi-interface yezigaba ezibini. , okunciphisa ukunyanzeliswa kobuso phakathi kwezi zigaba zimbini.Iimolekyuli ezininzi ze-surfactant (okanye ii-ion) zibhengezwa kumda wojongano, kokukhona kuncitshiswa kakhulu kuxinzelelo lobuso.
② Ezinye iimpawu ze-adsorption inwebu
Uxinzelelo lomphezulu we-adsorption membrane: I-adsorption ye-surfactant kwi-gas-liquid interface ukwenza inwebu ye-adsorption, efana nokubeka ishidi elidadayo elingenakususwa kujongano, iphepha elidadayo lityhala inwebu ye-adsorbent ecaleni komphezulu wesisombululo, kwaye inwebu ivelisa uxinzelelo. kwiphepha elidadayo, elibizwa ngokuba luxinzelelo lomphezulu.
I-Surface viscosity: Njengoxinzelelo lomphezulu, i-surface viscosity yipropathi eboniswa yi-insoluble molecular membrane.Ixhonywe ngentsimbi ecikizekileyo yentsimbi yeplatinum, ukuze inqwelomoya yayo iqhagamshelane nomphezulu wamanzi wetanki, ijikelezise iplatinum iringi, iplatinum iringi yi-viscosity yesithintelo samanzi, i-amplitude ibola ngokuthe ngcembe, ngokubhekiselele kwi-viscosity yomhlaba. kulinganisiwe.Indlela yile: okokuqala, uvavanyo luqhutywa kumphezulu wamanzi acocekileyo ukulinganisa ukubola kwe-amplitude, kwaye emva koko ukubola emva kokubunjwa kwenwebu yomhlaba kulinganiswa, kwaye i-viscosity ye-membrane yomhlaba ithathwe kumahluko phakathi kwezi zibini. .
I-surface viscosity inxulumene ngokusondeleyo nokuqina kwenwebu yomphezulu, kwaye ekubeni i-adsorption membrane inoxinzelelo lomphezulu kunye ne-viscosity, kufuneka ibe ne-elasticity.Ukuphakama koxinzelelo lomphezulu kunye nokuphakama kwe-viscosity ye-adsorbed membrane, iphezulu imodyuli yayo e-elastic.I-elastic modulus ye-membrane ye-adsorption surface ibalulekile kwinkqubo yokuzinzisa i-bubble.
③ Ukwenziwa kweemicelles
Izinyibiliko ezixutyiweyo ze-surfactants zithobela imithetho elandelwa zizisombululo ezifanelekileyo.Isixa se-surfactant adsorbed kumphezulu wesisombululo siyanda ngokuxinana kwesisombululo, kwaye xa ugxininiso lufikelela okanye ludlula kwixabiso elithile, isixa se-adsorption asisanyuki, kwaye ezi molekyuli zingaphezulu kwe-surfactant zikwisisombululo ngokuzenzekela. ngendlela okanye ngendlela eqhelekileyo.Zombini izenzo kunye nethiyori zibonisa ukuba benza imibutho kwisisombululo, kwaye le mibutho ibizwa ngokuba yi-micelles.
I-Critical Micelle Concentration (CMC): Ubuncinci boxinzelelo apho ii-surfactants zenza ii-micelles kwisisombululo kuthiwa lugxininiso olubalulekileyo lwe-micelle.
④ Amaxabiso e-CMC yee-surfactants eziqhelekileyo.

I-HLB sisishunqulelo se-hydrophile lipophile balance, ebonisa ibhalansi ye-hydrophilic kunye ne-lipophilic yamaqela e-hydrophilic kunye ne-lipophilic ye-surfactant, okt, ixabiso le-HLB le-surfactant.Ixabiso elikhulu le-HLB libonisa i-molecule ene-hydrophilicity enamandla kunye ne-lipophilicity ebuthakathaka;ngokuchaseneyo, i-lipophilicity eyomeleleyo kunye ne-hydrophilicity ebuthathaka.
① Amalungiselelo exabiso le-HLB
Ixabiso le-HLB lixabiso elihambelanayo, ngoko xa ixabiso le-HLB liphuhliswa, njengomgangatho, ixabiso le-HLB le-parafini wax, elingenayo i-hydrophilic properties, lichazwe ukuba libe yi-0, ngelixa ixabiso le-HLB le-sodium dodecyl sulfate, ngaphezulu kwe-soluble yamanzi, yi-40. Ngoko ke, ixabiso le-HLB le-surfactants ngokubanzi liphakathi koluhlu lwe-1 ukuya kwi-40. Ngokuqhelekileyo, i-emulsifiers kunye nexabiso le-HLB elingaphantsi kwe-10 lipophilic, ngelixa abo bangaphezu kwe-10 be-hydrophilic.Ngaloo ndlela, ukuguquka ukusuka kwi-lipophilic ukuya kwi-hydrophilic malunga ne-10.
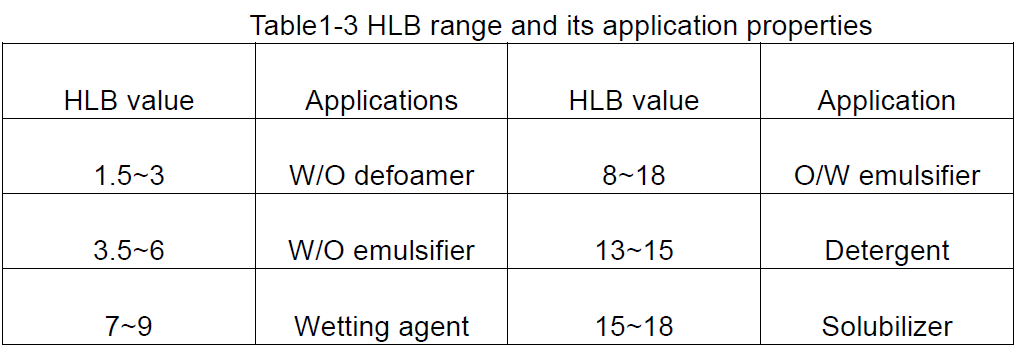
Ngokusekelwe kwimilinganiselo ye-HLB ye-surfactants, ingcamango eqhelekileyo yokusetyenziswa kwabo okunokwenzeka inokufumaneka, njengoko kuboniswe kwiThebhile 1-3.

Ulwelo ezimbini ezinganyibilikiyo, enye isasazwe kwelinye njengamasuntswana (amathontsi okanye iikristale ezingamanzi) zenza inkqubo ebizwa ngokuba yi-emulsion.Le nkqubo i-thermodynamically ingazinzile ngenxa yokwanda kwendawo yomda wezinto ezimanzi ezimbini xa i-emulsion isenziwa.Ukuze wenze i-emulsion izinzile, kuyimfuneko ukongeza icandelo lesithathu - i-emulsifier ukunciphisa amandla okudibanisa kwenkqubo.I-Emulsifier yeye-surfactant, umsebenzi wayo ophambili kukudlala indima ye-emulsion.Isigaba se-emulsion esikhoyo njengamaconsi sibizwa ngokuba yi-dispersed phase (okanye isigaba sangaphakathi, isigaba sokuyeka), kwaye esinye isigaba esidityaniswe kunye sibizwa ngokuba yi-dispersion medium (okanye isigaba sangaphandle, isigaba esiqhubekayo).
① Izinto zokuthambisa kunye neemulsions
I-emulsions eqhelekileyo, isigaba esinye ngamanzi okanye isisombululo esinamanzi, esinye isigaba zizinto eziphilayo ezingaxutywanga ngamanzi, njengegrisi, i-wax, njl njl. ihlakazekile emanzini ukwenza i-emulsion yohlobo lweoli emanzini, echazwe njenge-O / W (i-oyile / amanzi): amanzi ahlakazwe kwioli ukuze enze i-emulsion yohlobo lwe-oyile emanzini, echazwe njenge-W / O (amanzi / ioli).I-complex water-in-oil-in-water uhlobo lwe-W/O/W kunye ne-oyile-emanzini-kwi-oyile i-O/W/O uhlobo lwe-multi-emulsions nayo inokusekwa.
I-Emulsifiers isetyenziselwa ukuzinzisa i-emulsion ngokunciphisa ukuxinana kwe-interfacial kunye nokwenza i-membrane ye-interfacial membrane enye-molecule.
Kwi-emulsification yeemfuno ze-emulsifier:
a: I-emulsifier kufuneka ikwazi ukubhengeza okanye ukutyebisa i-interface phakathi kwezigaba ezibini, ukwenzela ukuba i-interfacial tension iyancipha;
b: I-emulsifier kufuneka inike amasuntswana kwintlawulo, ukwenzela ukuba i-electrostatic repulsion phakathi kwamasuntswana, okanye yenze i-stable, i-viscous ephezulu yokukhusela i-membrane ejikeleze amasuntswana.
Ke ngoko, into esetyenziswa njenge-emulsifier kufuneka ibe namaqela e-amphiphilic ukuze i-emulsify, kwaye i-surfactants inokuhlangabezana nale mfuneko.
② Ukulungiselela iindlela ze-emulsions kunye nezinto ezichaphazela ukuzinza kwe-emulsion
Kukho iindlela ezimbini zokulungiselela i-emulsions: enye kukusebenzisa indlela yomatshini ukusabalalisa ulwelo kwiincinci ezincinci kwelinye ulwelo, olusetyenziswa kakhulu kwishishini ukulungiselela i-emulsions;enye kukunyibilikisa ulwelo kwimo yemolekyuli kolunye ulwelo, kwaye emva koko ulwenze luqokelelene ngokufanelekileyo ukwenza ii-emulsions.
Ukuzinza kwe-emulsion kukukwazi ukulwa ne-particle aggregation ekhokelela ekuhlukaneni kwesigaba.Ii-emulsions ziinkqubo ezingazinzanga ze-thermodynamically ezinamandla amakhulu asimahla.Ngoko ke, okubizwa ngokuba yi-stability of an emulsion ngokwenene lixesha elifunekayo ukuze inkqubo ifikelele kwi-equilibrium, oko kukuthi, ixesha elifunekayo lokwahlula enye ye-liquids kwinkqubo ukuba yenzeke.
Xa inwebu yangaphakathi eneealkoholi ezinamafutha, iiasidi ezinamafutha kunye neeamine ezinamafutha kunye nezinye iimolekyuli zepolar eziphilayo, amandla enwebu aphezulu kakhulu.Oku kungenxa yokuba, kwi-interfacial adsorption layer yeemolekyuli ze-emulsifier kunye ne-alcohols, i-acids kunye ne-amines kunye nezinye iimolekyuli ze-polar ukwenza "i-complex", ukwenzela ukuba i-interfacial inwebu yamandla yanda.
Ii-emulsifiers ezibandakanya ngaphezu kwe-surfactants ezimbini zibizwa ngokuba zi-emulsifiers ezixubileyo.Umxube we-emulsifier odityanisiweyo kujongano lwamanzi/oyile;isenzo se-intermolecular sinokwenza ii-complexes.Ngenxa yesenzo esinamandla se-intermolecular, ukunyanzeliswa kwe-interfacial kuncitshiswe kakhulu, inani le-emulsifier adsorbed kwi-interface yanda kakhulu, ukubunjwa kobuninzi be-membrane ye-interfacial kwandisa, amandla ayanda.
Umrhumo we-liquid beads unempembelelo ebalulekileyo ekuzinzeni kwe-emulsion.Ii-emulsions ezizinzile, ezinamaso olwelo ahlawuliswa ngokubanzi.Xa i-ionic emulsifier isetyenzisiwe, i-ion emulsifier adsorbed kwi-interface ineqela layo le-lipophilic elifakwe kwisigaba seoli kwaye iqela le-hydrophilic likwisigaba samanzi, ngaloo ndlela lenza amaso olwelo ahlawuliswe.Njengoko amaso emulsion kunye nentlawulo efanayo, bayagxotha omnye komnye, akulula ukuba agglomerate, ukwenzela ukuba ukuzinza kwandiswe.Kungabonwa ukuba i-ion emulsifier ibhengezwa kumaso, intlawulo enkulu, amandla amakhulu okuthintela amaso ukusuka kwi-agglomeration, inkqubo ye-emulsion izinzile.
I-viscosity ye-emulsion dispersion medium inempembelelo ethile ekuzinzeni kwe-emulsion.Ngokuqhelekileyo, i-viscosity ephezulu ye-dispersion medium, iphezulu ukuzinza kwe-emulsion.Oku kungenxa yokuba i-viscosity ye-dispersion medium inkulu, enefuthe elinamandla kwi-Brownian motion ye-liquid beads kwaye inciphisa ukungqubana phakathi kwamaso olwelo, ukwenzela ukuba inkqubo ihlale izinzile.Ngokuqhelekileyo, izinto ze-polymer ezinokuchithwa kwi-emulsions zinokunyusa i-viscosity yenkqubo kwaye zenze ukuzinza kwee-emulsions kuphezulu.Ukongeza, iipolymers zinokwenza i-membrane eyomeleleyo ye-interfacial, eyenza inkqubo ye-emulsion izinze ngakumbi.
Kwezinye iimeko, ukongezwa komgubo oqinileyo kunokwenza ukuba i-emulsion ithande ukuzinza.Umgubo oqinileyo usemanzini, i-oyile okanye i-interface, ngokuxhomekeke kwi-oyile, amanzi kumthamo wokumanzisa womgubo oqinileyo, ukuba umgubo oqinileyo awumanzi ngokupheleleyo ngamanzi, kodwa kwakhona umanzi ngeoli, uya kuhlala phezu kwamanzi kunye neoli. ujongano.
Umgubo oqinileyo awuyenzi i-emulsion iqine ngenxa yokuba umgubo oqokelelwe kwi-interface uphucula i-membrane ye-interfacial, efana ne-adsorption ye-interfacial adsorption ye-emulsifier molecules, ngoko ke ngokusondeleyo i-powder eqinile icwangciswa kwi-interface, iqinile ngakumbi. emulsion yiyo.
I-surfactants inamandla okwandisa kakhulu ukunyibilika kwezinto eziphilayo ezinganyibilikiyo okanye ezinganyibilikiyo kancinane emanzini emva kokwenza iimicelles kwisisombululo esinamanzi, kwaye isisombululo sisobala ngeli xesha.Esi siphumo se-micelle sibizwa ngokuba yi-solubilization.I-surfactant enokuvelisa i-solubilization ibizwa ngokuba yi-solubilizer, kwaye i-organic matter enyibilikayo ibizwa ngokuba yi-solubilized matter.

I-Foam idlala indima ebalulekileyo kwinkqubo yokuhlamba.I-Foam yinkqubo yokusasazwa apho irhasi isasazwa kulwelo okanye okuqinileyo, kunye nerhasi njengenqanaba elisasaziweyo kunye nolwelo okanye oluqinileyo njengesixhobo sokuchithachitha, le yangaphambili ibibizwa ngokuba yigwebu elulwelo, ngelixa le yokugqibela ibizwa ngokuba yi-foam eqinileyo, njengeplastiki enogwebu, iglasi enogwebu, isamente enogwebu njl.
(1) Ukwenziwa kogwebu
Xa sithetha ngogwebu apha sithetha i-aggregate yamaqamza omoya ahlulwe yinwebu yolwelo.Olu hlobo lweqamza luhlala luphakama ngokukhawuleza kumphezulu wolwelo ngenxa yomahluko omkhulu woxinaniso phakathi kwesigaba esisasaziweyo (igesi) kunye ne-dispersion medium (ulwelo), kudityaniswe ne-viscosity ephantsi yolwelo.
Inkqubo yokwenza iqamza kukuzisa isixa esikhulu serhasi kulwelo, kwaye amaqamza akulwelo abuyela ngokukhawuleza kumphezulu, enze i-aggregate yamaqamza ahlulwe sisixa esincinci segesi engamanzi.
Igwebu lineempawu ezimbini ezibalulekileyo ngokwemorphology: enye yeyokuba amaqamza njengesigaba esisasaziweyo ahlala emile okwepolyhedral, oku kungenxa yokuba ekudibaneni kwamaqamu, kukho utyekelo lokuba ifilimu elulwelo ibe bhityile ukuze amaqamza abe mncinci. i-polyhedral, xa ifilimu yolwelo iyancipha ukuya kwinqanaba elithile, ikhokelela ekuqhekekeni kwe-bubble;okwesibini kukuba ulwelo olusulungekileyo alukwazi ukwenza i-foam ezinzileyo, ulwelo olunokwenza i-foam lunamacandelo amabini okanye ngaphezulu.Izisombululo ezinamanzi ze-surfactants ziqhelekileyo kwiinkqubo ezithanda ukuveliswa kwegwebu, kwaye amandla abo okuvelisa amagwebu anxulumene nezinye iipropati.
Ii-surfactants ezinamandla atyhobozayo zibizwa ngokuba zii-agent ezinegwebu.Nangona i-agent ye-foam inekhono elihle le-foam, kodwa i-foam eyenziweyo ayinakukwazi ukugcina ixesha elide, oko kukuthi, ukuzinza kwayo akukho nto ilungileyo.Ukuze kugcinwe ukuzinza kwe-foam, ngokuphindaphindiweyo kwi-agent e-foam yokongeza izinto ezinokunyusa ukuzinza kwe-foam, into ebizwa ngokuba yi-foam stabilizer, i-stabilizer esetyenziswa ngokuqhelekileyo i-lauryl diethanolamine kunye ne-dodecyl dimethylamine oxide.
(2) Ukuzinza kwegwebu
I-Foam yinkqubo engazinzanga ye-thermodynamically kwaye intsingiselo yokugqibela kukuba indawo epheleleyo yolwelo ngaphakathi kwenkqubo iyancipha emva kokuba i-bubble yaphukile kwaye amandla asimahla ayancipha.Inkqubo yokukhupha amagwebu yinkqubo apho inwebu yolwelo eyahlula irhasi iba ngqindilili kwaye ibhitye de iqhawuke.Ngoko ke, iqondo lokuzinza kwe-foam lixhomekeke ngokukodwa kwisantya sokukhutshwa kwamanzi kunye namandla efilimu yolwelo.Ezi zinto zilandelayo nazo zinefuthe koku.
(3) Ukutshatyalaliswa kwegwebu
Umgaqo osisiseko wokutshatyalaliswa kwe-foam kukutshintsha iimeko ezivelisa i-foam okanye ukuphelisa izinto zokuzinzisa i-foam, ngoko ke kukho iindlela zombini kunye neekhemikhali zokungcolisa.
Ukunciphisa umzimba kuthetha ukutshintsha iimeko zokuvelisa i-foam ngelixa ugcina ukubunjwa kweekhemikhali zesisombululo se-foam, njengokuphazamiseka kwangaphandle, utshintsho kwiqondo lokushisa okanye uxinzelelo kunye nonyango lwe-ultrasonic zonke iindlela ezisebenzayo zomzimba zokuphelisa i-foam.
Indlela yekhemikhali yokukhupha amagwebu kukongeza izinto ezithile zokunxibelelana ne-agent yogwebu ukunciphisa amandla efilimu engamanzi kwi-foam kwaye ngaloo ndlela kuncitshiswe ukuzinza kwe-foam ukufezekisa injongo ye-defoaming, ezo zinto zibizwa ngokuba yi-defoamers.Uninzi lwee-defoamers zi-surfactants.Ke ngoko, ngokwendlela yokwenza i-defoamer, i-defoamer kufuneka ibe namandla anamandla okunciphisa ukuxinezeleka komphezulu, kulula ukubhengeza kumphezulu, kwaye ukusebenzisana phakathi kweeathomu ze-adsorption zibuthathaka, iimolekyuli ze-adsorption zilungelelaniswe kwisakhiwo esikhululekileyo.
Kukho iintlobo ezahlukeneyo ze-defoamer, kodwa ngokusisiseko, zonke azizo-ionic surfactants.Ii-non-ionic surfactants zineepropathi zokuchasa ugwebu kufutshane okanye ngaphezulu kwendawo yazo yelifu kwaye zihlala zisetyenziswa njenge-defoamers.Utywala, ngokukodwa i-alcohols enesakhiwo se-branching, i-fatty acids kunye ne-fatty acid esters, i-polyamides, i-phosphate esters, i-oyile ye-silicone, njl.
(4) Ugwebu kunye nokuhlamba
Akukho nxibelelwano oluthe ngqo phakathi kwe-foam kunye nokusebenza kokuhlamba kunye nomthamo we-foam awubonisi ukusebenza kokuhlamba.Umzekelo, ii-nonionic surfactants zineepropathi zogwebu ezincinci kunesepha, kodwa ukucocwa kwazo kubhetele kakhulu kunesepha.
Kwezinye iimeko, i-foam inokuba luncedo ekususeni ukungcola kunye nokungcola.Ngokomzekelo, xa uhlamba izitya ekhaya, i-foam ye-detergent ithatha amathontsi yeoli kwaye xa ukhuhla iikhaphethi, i-foam inceda ukuchola uthuli, umgubo kunye nolunye ukungcola okuqinileyo.Ukongeza, i-foam ngamanye amaxesha ingasetyenziswa njengesalathiso sokusebenza kwe-detergent.Ngenxa yokuba i-oyile enamafutha anefuthe elivimbelayo kwi-foam ye-detergent, xa kukho ioli eninzi kunye ne-detergent encinci, akukho foam iya kuveliswa okanye i-foam yasekuqaleni iya kunyamalala.I-Foam inokuthi ngamanye amaxesha isetyenziswe njengesalathisi sokucoceka kwe-rinse, njengoko inani le-foam kwisisombululo se-rinse livame ukuhla ngokunciphisa i-detergent, ngoko ke inani le-foam lingasetyenziselwa ukuvavanya iqondo lokuhlanjululwa.
Ngomqondo obanzi, ukuhlamba yinkqubo yokususa amacandelo angafunekiyo kwizinto eziza kuhlamba kunye nokufezekisa injongo ethile.Ukuhlamba ngendlela eqhelekileyo kubhekisela kwinkqubo yokususa ukungcola ebusweni bomthwali.Ekuhlanjweni, ukusebenzisana phakathi kokungcola kunye nomthwali kuyancipha okanye kupheliswe yisenzo sezinto ezithile zeekhemikhali (umz., i-detergent, njl.), ukwenzela ukuba ukudibanisa ukungcola kunye nomthwali kuguqulwe kwindibaniselwano yokungcola kunye ne-detergent, kwaye ekugqibeleni ukungcola kwahlulwa kumthwali.Njengoko izinto eziza kuhlamba kunye nokungcola okuza kususwa zihlukeneyo, ukuhlamba kuyinkqubo enzima kakhulu kwaye inkqubo esisiseko yokuhlamba ingabonakaliswa kolu budlelwane bulula.
Carrie··Ubumdaka + Isicoci= Umthwali + Ukungcola · Isicoci
Inkqubo yokuhlamba ngokuqhelekileyo inokwahlulwa ibe ngamanqanaba amabini: okokuqala, phantsi kwesenzo se-detergent, ukungcola kuyahlukana nomphathi wayo;okwesibini, ukungcola okufihliweyo kuyahlakazwa kwaye kumiswe phakathi.Inkqubo yokuhlamba yinkqubo yokubuyisela umva kwaye ukungcola okuchithwa kwaye kunqunyanyiswe phakathi kunokuphinda kuhlanjwe kwakhona ukusuka phakathi ukuya kwinto ehlanjwayo.Ngoko ke, i-detergent efanelekileyo kufuneka ibe nekhono lokusabalalisa kunye nokumisa ukungcola kunye nokuthintela ukubuyiswa kwakhona kokungcola, ngaphezu kokukwazi ukususa ukungcola kumthwali.
(1) Iintlobo zobumdaka
Nakwinto enye, uhlobo, ukubunjwa kunye nobungakanani bobumdaka bunokwahluka ngokuxhomekeke kwindawo esetyenziswa kuyo.Ukungcola komzimba we-oyile ubukhulu becala zezinye ioyile zezilwanyana kunye nemifuno kunye nee-oyile zeminerali (ezifana ne-oyile ekrwada, i-oyile yamafutha, itela yamalahle, njl.njl.), ubumdaka obuqinileyo ikakhulu ngumle, uthuthu, irusi, i-carbon emnyama, njl njl. Ngokubhekiselele kukungcola kwempahla, kukho ukungcola emzimbeni womntu, njengokubila, i-sebum, igazi, njl.;ukungcola kokutya, okufana namabala eziqhamo, amabala e-oyile yokupheka, amabala e-condiment, istatshi, njl.;ukungcola okuvela kwizinto zokuthambisa, ezifana ne-lipstick, i-nail polish, njl.;ubumdaka obuvela kwiatmosfera, njengothuthu, uthuli, udaka, njalo njalo;abanye, njenge-inki, iti, ukutyabeka, njl. Iza kwiindidi ezahlukeneyo.
Iindidi ezahlukeneyo zokungcola zinokwahlulwa zibe ziindidi ezintathu eziphambili: ubumdaka obuqinileyo, ubumdaka obumanzi kunye nobumdaka obukhethekileyo.
① Ubumdaka obuqinileyo
Ukungcola okuqhelekileyo okuqinileyo kubandakanya amasuntswana othuthu, udaka, umhlaba, umhlwa kunye nekhabhoni emnyama.Uninzi lwala masuntswana anentlawulo yombane kumphezulu wawo, uninzi lwazo luhlawuliswa kakubi kwaye lunokuthengiswa ngokulula kwizinto zefiber.Ukungcola okuqinileyo ngokuqhelekileyo kunzima ukunyibilika emanzini, kodwa kunokuchithwa kwaye kunqunyanyiswe ngezisombululo zokucoca.Ukungcola okuqinileyo kunye nenqaku elincinci lobunzima kunzima kakhulu ukukususa.
② Ubumdaka obumanzi
Ukungcola kolwelo kuninzi kwi-oyile-enyibilikayo, kubandakanywa i-oyile yezityalo kunye nezilwanyana, i-fatty acids, i-alcohols ezinamafutha, i-oyile yamaminerali kunye nee-oxides zazo.Phakathi kwazo, i-oyile yezityalo kunye nezilwanyana, i-fatty acids kunye ne-alkali saponification ingenzeka, ngelixa i-alcohols enamafutha, i-oyile yamaminerali ayinayo i-saponified yi-alkali, kodwa inokunyibilika kwi-alcohols, i-ethers kunye ne-hydrocarbon organic solvents, kunye ne-detergent yamanzi isisombululo se-emulsification kunye nokusabalalisa.Ubumdaka obumanzi obunyibilikayo kwi-oyile bunamandla awomeleleyo anezinto zefayibha, kwaye bubonakala buqina ngakumbi kwiintsinga.
③ Ukungcola okukhethekileyo
Ukungcola okukhethekileyo kubandakanya iiprotheyini, isitashi, igazi, iimfihlo zabantu ezifana nokubila, i-sebum, umchamo kunye nejusi yeziqhamo kunye nejusi yeti.Uninzi lolu hlobo lobumdaka lunokubhengezwa ngokwemichiza kwaye ngamandla kwizinto zefiber.Ngoko ke, kunzima ukuhlamba.
Iindidi ezahlukeneyo zokungcola azifane zifumaneke zodwa, kodwa zihlala zixutywa kunye kwaye zibhengezwa kwinto leyo.Ukungcola ngamanye amaxesha kunokuba ne-oxidized, ukubola okanye ukubola phantsi kweempembelelo zangaphandle, ngaloo ndlela kudala ukungcola okutsha.
(2) Ukuncamathela ubumdaka
Impahla, izandla, njl.Ukungcola kubambelela kwizinto ngeendlela ezahlukeneyo, kodwa akukho ngaphezu kokubambelela ngokomzimba kunye neekhemikhali.
① Ukuncamathelwa komsizi, uthuli, udaka, isanti kunye namalahle empahleni kukuncamathela ngokwasemzimbeni.Ngokuqhelekileyo, ngokunamathela kokungcola, kunye nendima phakathi kwento enebala ibuthathaka, ukususwa kokungcola kulula.Ngokwamandla ahlukeneyo, ukunamathela komzimba wokungcola kunokwahlulwa kumatshini wokubambelela kunye nokunamathela kwe-electrostatic.
A: Ukubambelela koomatshini
Olu hlobo lokuncamathelisa ikakhulu lubhekiselele ekuncamatheleni kobumdaka obuqinileyo (umz., uthuli, udaka kunye nesanti).I-Mechanical adhesion enye yeendlela ezibuthathaka zokubambelela ukungcola kwaye zinokususwa phantse ngeendlela ezichanekileyo, kodwa xa ukungcola kuncinci (<0.1um), kunzima ukususa.
B: Ukubambelela kwi-Electrostatic
Ukubambelela kwi-electrostatic kubonakaliswa ikakhulu kwisenzo sokungcola okuhlawuliswayo kwizinto ezichaseneyo.Izinto ezininzi ezineefayibha zihlawuliswa kakubi emanzini kwaye zinokubambelela ngokulula kukungcola okuchajiwe kakuhle, okufana neentlobo zekalika.Obunye ubumdaka, nangona buhlawulwe kakubi, njengamasuntswana ekhabhoni emnyama kwizisombululo ezinamanzi, zinokubambelela kwiintsinga ngokusebenzisa iibhulorho ze-ionic (iionini phakathi kwezinto ezininzi ezichajiweyo ezichaseneyo, zisebenza kunye nazo ngendlela efana nebhulorho) ezenziwe ziiyoni ezilungileyo emanzini (umz. , Ca2+, Mg2+ njl.).
Intshukumo ye-electrostatic yomelele kunesenzo esilula soomatshini, nto leyo eyenza ukususwa kobumdaka kube nzima.
② Ukunamathela kwimichiza
Ukunamathela kwimichiza kubhekiselele kwinto yokungcola esebenza entweni ngemichiza okanye iibhondi zehydrogen.Ngokomzekelo, ubumdaka obuqinileyo be-polar, iprotheni, i-rust kunye nezinye izinto zokubambelela kwizinto zefiber, iifibers ziqukethe i-carboxyl, i-hydroxyl, i-amide kunye namanye amaqela, la maqela kunye ne-oily dirty fatty acids, i-alcohols ezinamafutha kulula ukwenza iibhondi ze-hydrogen.Amandla ekhemikhali anamandla ngokubanzi kwaye ukungcola ke ngoko kunamathele ngokuqinileyo kwinto.Olu hlobo lokungcola kunzima ukususa ngeendlela eziqhelekileyo kwaye lufuna iindlela ezikhethekileyo zokujongana nalo.
Iqondo lokubambelela kokungcola lihambelana nobume bobumdaka ngokwawo kunye nohlobo lwento ehambelana nayo.Ngokuqhelekileyo, amasuntswana abambelela ngokulula kwizinto ezinefiber.Ubuncinci ubuncinci bobumdaka obuqinileyo, ukuqina kokuqina.Ukungcola kwepolar kwizinto ze-hydrophilic ezifana ne-cotton kunye neglasi zibambelela kakhulu kunokungcola okungeyona i-polar.Ubumdaka obungeyonxalenye yencam yomhlaba bubambelela ngamandla kunobumdaka obuvela kwincam yomhlaba, obufana namafutha encam, uthuli nodongwe, kwaye akulula ukubususa nokucoca.
(3) Indlela yokususa ukungcola
Injongo yokuhlamba kukususa ukungcola.Kwiqondo lobushushu elithile (ingakumbi amanzi).Ukusebenzisa iziphumo ezahlukeneyo zomzimba kunye neekhemikhali ze-detergent ukwenza buthathaka okanye ukuphelisa umphumo wokungcola kunye nezinto ezihlanjiweyo, phantsi kwesenzo samandla athile omatshini (njengokuxutywa kwezandla, ukuxubha umatshini wokuhlamba, impembelelo yamanzi), ukwenzela ukuba ukungcola kunye nezinto ezihlanjiweyo. ukusuka kwinjongo yokucoca.
① Indlela yokususa ubumdaka obumanzi
A: Ukumanzisa
Ukungcoliswa kolwelo ikakhulu kusekwe kwi-oyile.I-oyile ingcolisa izinto ezimanzi ezininzi kwaye isasazeke ngaphezulu okanye ngaphantsi njengefilimu yeoli kumphezulu wezinto ezinentsinga.Inyathelo lokuqala kwisenzo sokuhlamba kukumanzisa umphezulu ngolwelo lokuvasa.Ukwenza umzekeliso, umphezulu wosinga kunokucingelwa njengomgangatho oqinileyo ogudileyo.
B: I-Oil detachment-curling mechanism
Isinyathelo sesibini kwisenzo sokuhlamba kukukhutshwa kweoli kunye negrisi, ukukhutshwa kokungcola kolwelo kufezekiswa ngohlobo lokudibanisa.Ubumdaka obumanzi bebukhona ekuqaleni kumphezulu ngendlela yefilimu yeoyile esasaziweyo, kwaye phantsi kwefuthe elikhethekileyo lokumanzisa ulwelo lokuhlambela kwindawo eqinileyo (oko kukuthi, umphezulu wefiber), yazisonga yaba ngamaso e-oyile inyathelo ngenyathelo, leyo zathatyathelwa indawo lulwelo lokuvasa kwaye ekugqibeleni zawushiya umphezulu phantsi kwamandla athile angaphandle.
② Indlela yokususa ubumdaka obuqinileyo
Ukususwa kokungcola kolwelo ikakhulu ngokuchetywa okukhethiweyo komthuthi wobumdaka ngesisombululo sokuhlamba, ngelixa indlela yokususa ubumdaka obuqinileyo yahlukile, apho inkqubo yokuhlamba ikakhulu malunga nokumanzisa ubunzima bokungcola kunye nomphambili wayo othwala ngokuhlamba. isisombululo.Ngenxa ye-adsorption ye-surfactants kumdaka oqinileyo kunye nomphezulu wayo ophetheyo, intsebenziswano phakathi kokungcola kunye nomphezulu kuyancitshiswa kwaye amandla okunamathela obunzima bokungcola kumphezulu ayancipha, ngaloo ndlela ubunzima bokungcola bususwa ngokulula kumphezulu. umthwali.
Ukongeza, i-adsorption ye-surfactants, ngakumbi i-ionic surfactants, kumphezulu wobumdaka obuqinileyo kunye nomthwali wayo unakho ukonyusa amandla angaphezulu kumphezulu wobumdaka obuqinileyo kunye nomthwali wayo, onceda ngakumbi ekususweni kokungcola. ubumdaka.Imiphezulu eqinileyo okanye enentsinga ngokubanzi idla ngokuhlawuliswa kakubi kumajelo osasazo anamanzi kwaye ke ngoko inokwenza izaleko eziphindwe kabini zombane kubunzima bobumdaka okanye umphezulu oqinileyo.Ngenxa yokunyanzeliswa kweentlawulo ze-homogeneous, ukunamathela kweengqungquthela zokungcola emanzini kwindawo eqinile kuyancipha.Xa i-surfactant ye-anionic yongezwa, kuba inokunyusa kwangaxeshanye amandla angalunganga omgangatho wobumdaka kunye nomgangatho oqinileyo, ukunyanyeka phakathi kwabo kuphuculwe ngakumbi, amandla okubambelela kwiqhekeza ayancipha ngakumbi, kwaye ukungcola kulula ukukususa. .
I-non-ionic surfactants i-adsorbed kumphezulu oqinileyo ohlawuliswayo ngokubanzi kwaye nangona ingatshintshi kakhulu i-interface enokubakho, i-adsorbed non-ionic surfactants ikholisa ukwenza ubukhulu obuthile bomaleko we-adsorbed kumphezulu onceda ukuthintela ukubuyiswa kobumdaka.
Kwimeko ye-cationic surfactants, i-adsorption yabo iyanciphisa okanye ikhuphe amandla angalunganga obuninzi bokungcola kunye nomgangatho wayo ophetheyo, okunciphisa ukunyanzeliswa phakathi kokungcola kunye nomgangatho kwaye ngoko ke akuhambisani nokususwa kokungcola;ngaphezu koko, emva kokufakwa kwi-adsorption kumphezulu oqinileyo, i-cationic surfactants ikholisa ukujika umphezulu oqinileyo we-hydrophobic kwaye ke ngoko akwenzeli ukumanzisa komphezulu kwaye ke ngoko ukuvaswa.
③ Ukususwa kwemihlaba ekhethekileyo
Iiprotheyini, isitashi, iimfihlo zabantu, ijusi yesithelo, ijusi yeti kunye nolunye ukungcola okunjalo kunzima ukukususa kunye ne-surfactants eqhelekileyo kwaye kufuna unyango olukhethekileyo.
Amabala eprotheyini afana nekhrimu, amaqanda, igazi, ubisi kunye nelindle lolusu lithanda ukujiya kwimicu kunye nokuwohloka kwaye libambelele ngakumbi.Ukungcoliswa kweeprotheyini kunokususwa ngokusebenzisa iiproteases.I-enzyme ye-protease iqhekeza iiprotheni ezisebumdaka zibe yi-amino acids enyibilikayo emanzini okanye i-oligopeptides.
Amabala esitatshi aphuma ikakhulu ekutyeni, okunye okunje ngegravy, iglu, njl.njl. I-Amylase inefuthe le-catalytic kwi-hydrolysis ye-starch stains, ebangela ukuba isitashi siphuke sibe yiswekile.
I-Lipase ibangela ukubola kwe-triglycerides, ekunzima ukuyisusa ngeendlela eziqhelekileyo, ezifana ne-sebum kunye namafutha adliwayo, kwaye iwaphule kwi-glycerol e-soluble kunye ne-fatty acids.
Amanye amabala anemibala evela kwijusi yeziqhamo, iijusi zeti, i-inki, i-lipstick njl njl.La mabala anokususwa ngokuphendula kwe-redox kunye ne-oxidizing okanye i-agent yokunciphisa njenge-bleach, eyonakalisa isakhiwo samaqela avelisa umbala okanye ancedisa umbala kunye nokuwanciphisa abe ngamacandelo amancinci anyibilikayo emanzini.
(4) Indlela yokususa ibala yokucoca okomileyo
Oku kungasentla ngenene ngamanzi njengendawo yokuhlamba.Enyanisweni, ngenxa yeentlobo ezahlukeneyo zempahla kunye nesakhiwo, ezinye iimpahla zisebenzisa ukuhlamba kwamanzi azilungelekanga okanye akulula ukuzihlamba zicocekile, ezinye iimpahla emva kokuhlamba kunye nokuguqulwa, ukubola, njl. kulula ukuvuvukala, kwaye yomile kwaye kulula ukuyicutha, ngoko emva kokuhlamba kuya kuphazamiseka;ngokuhlamba iimveliso zoboya kwakhona kaninzi kubonakala shrinkage phenomenon, ezinye iimveliso zoboya kunye nokuhlamba amanzi kwakhona kulula pilling, utshintsho umbala;Ezinye iisilika ziziva zisiba mbi emva kokuba zihlanjiwe kwaye ziphulukene nokubengezela kwazo.Kwezi mpahla zihlala zisebenzisa indlela yokucoca okomileyo ukuze ingcolise.Okubizwa ngokuba kucocekile okomileyo ngokuqhelekileyo kubhekisela kwindlela yokuhlamba kwi-solvents ye-organic, ngokukodwa kwi-solvents non-polar.
Ukucoca okomileyo yindlela ethambileyo yokuvasa kunokuvasa amanzi.Ngenxa yokuba ukucoca okomileyo akudingi isenzo esininzi soomatshini, akubangeli umonakalo, ukushwabana kunye nokuguqulwa kwempahla, ngelixa ii-agent zokucoca ezomileyo, ngokungafaniyo namanzi, azifane zivelise ukwanda kunye nokunciphisa.Ngethuba nje iteknoloji iphathwa ngokufanelekileyo, iimpahla zinokomiswa ngaphandle kokuphazamiseka, umbala ophelileyo kunye nobomi benkonzo eyandisiweyo.
Ngokumalunga nokucoca okomileyo, kukho iintlobo ezintathu ezibanzi zokungcola.
① Ukungcola okunyibilikayo kwi-oyile Ukungcola okunyibilikayo kwi-oyile kubandakanya zonke iintlobo zeoyile kunye negrisi, enolwelo okanye egreasy kwaye inokunyibilika kwizinyibilikisi ezomileyo zokucoca.
②Ubumdaka obunyibilikayo ngamanzi Ukungcola okunyibilikayo ngamanzi kunyibilika kwizisombululo ezinamanzi, kodwa hayi kwizixhobo zokucoca ezomileyo, kubhengezwa kwiimpahla ezikwimo enamanzi, amanzi aba ngumphunga emva kwemvula yezinto eziqinileyo zegranular, ezinjengeetyuwa ezingaphiliyo, istatshi, iiprotheyini, njl.
③I-oyile kunye namanzi amdaka anganyibilikiyo Ioyile kunye nobumdaka obunganyibilikiyo namanzi ayinyibiliki emanzini kwaye ayinakunyibilika kwizinyibilikisi zokucoca ezomileyo, ezinje ngekhabhoni emnyama, iisilicate zesinyithi ezahlukeneyo kunye neeoksidi, njl.
Ngenxa yobume beentlobo ezahlukeneyo zokungcola, kukho iindlela ezahlukeneyo zokususa ukungcola kwinkqubo yokucoca okomileyo.Imihlaba enyibilikayo yeoli, efana neoyile yezilwanyana kunye nemifuno, ioyile yamaminerali kunye negrisi, inyibilika ngokulula kwi-solvents yezinto eziphilayo kwaye inokususwa ngokulula ekucoceni okomileyo.Ukunyibilika okugqwesileyo kwezinyibilikisi zokucoca okomileyo kwiioyile kunye namafutha zivela kumandla e-van der Walls phakathi kweeathom.
Ukususwa kokungcola okunyibilikayo kwamanzi okufana neetyuwa ezingabonakaliyo, iiswekile, iiprotheni kunye nokubila, umlinganiselo ofanelekileyo wamanzi kufuneka wongezwe kwi-agent yokucoca okomileyo, ngaphandle koko ukungcola kwamanzi okunyibilikayo kunzima ukukususa kwimpahla.Nangona kunjalo, amanzi kunzima ukunyibilika kwi-ejenti yokucoca okomileyo, ngoko ke ukwandisa umthamo wamanzi, kufuneka kwakhona ungeze i-surfactants.Ubukho bamanzi kwi-ejenti yokucoca i-dry-clean-clean-clean-desktop ingenza umphezulu wokungcola kunye neengubo zifakwe emanzini, ukwenzela ukuba kube lula ukusebenzisana namaqela e-polar of surfactants, ehambelana ne-adsorption of surfactants phezu komhlaba.Ukongeza, xa ii-surfactants zenza ii-micelles, ubumdaka obunyibilikayo emanzini kunye namanzi zinokunyibilika kwiimicelles.Ukongeza ekunyuseni umthamo wamanzi we-solvent eyomileyo yokucoca, i-surfactants nayo inokudlala indima ekuthinteleni ukuphinda kufakwe ukungcola ukunyusa umphumo wokutshatyalaliswa.
Ubukho bamanzi amancinci buyimfuneko ukususa ukungcola kwamanzi, kodwa amanzi amaninzi anokubangela ukuphazamiseka kunye nokushwabana kwezinye iimpahla, ngoko ke inani lamanzi kwi-ejenti yokucoca elomileyo kufuneka libe liphakathi.
Ukungcola okungeyona i-soluble yamanzi okanye i-oil-soluble, iincinci eziqinileyo ezifana nomlotha, udaka, umhlaba kunye nekhabhoni emnyama, ngokuqhelekileyo ifakwe kwisambatho ngamandla e-electrostatic okanye ngokudibanisa neoli.Ekucoceni okomileyo, ukuhamba kwe-solvent, impembelelo inokwenza i-electrostatic force adsorption of dirty off, kunye ne-ejenti yokucoca eyomileyo inokunyibilikisa ioli, ukwenzela ukuba indibaniselwano yeoli kunye nokungcola kwaye ifakwe kwiingubo zamasuntswana aqinileyo kwindawo eyomileyo. -i-agent yokucoca, i-ejenti yokucoca eyomileyo kwisixa esincinci samanzi kunye ne-surfactants, ukwenzela ukuba abo baphuma kwiingqungquthela zokungcola eziqinileyo zikwazi ukuzinza ukumiswa, ukusabalalisa, ukuthintela ukufakwa kwayo kwakhona kwempahla.
(5) Izinto ezichaphazela isenzo sokuhlamba
I-adsorption ye-adsorption ye-surfactants kwi-interface kunye nokunciphisa umphezulu (i-interfacial) ingcinezelo yizona zinto ziphambili ekususweni kokungcola okumanzi okanye okuqinileyo.Nangona kunjalo, inkqubo yokuhlamba iyinkimbinkimbi kwaye umphumo wokuhlamba, kunye nohlobo olufanayo lwe-detergent, luphenjelelwa ngezinye izinto ezininzi.Ezi zinto ziquka ukugxilwa kwe-detergent, ubushushu, ubunjani bomhlaba, uhlobo lwefiber kunye nesakhiwo selaphu.
① Ugxininiso lwe-surfactant
Iimicelles ze-surfactants kwisisombululo zidlala indima ebalulekileyo kwinkqubo yokuhlamba.Xa ugxininiso lufikelela kugxininiso olubalulekileyo lwe-micelle (CMC), umphumo wokuhlamba ukhula ngokukhawuleza.Ngoko ke, ukuxinwa kwe-detergent kwi-solvent kufuneka ibe phezulu kunexabiso le-CMC ukuze libe nefuthe elihle lokuhlamba.Nangona kunjalo, xa uxinaniso lwe-surfactant luphezulu kunexabiso le-CMC, ukonyuka okonyuka kwesiphumo sokuvasa akucacanga kwaye akukho mfuneko yokunyusa uxinzelelo lwe-surfactant kakhulu.
Xa ususa ioyile nge-solubilization, isiphumo se-solubilization siyanyuka ngokunyuka koxinzelelo lwe-surfactant, nokuba ugxininiso lungaphezulu kwe-CMC.Ngeli xesha, kuyacetyiswa ukuba kusetyenziswe isepha kwindawo esembindini yendawo.Ngokomzekelo, ukuba kukho ukungcola okuninzi kwi-cuffs kunye nekhola yengubo, i-detergent ingasetyenziselwa ngexesha lokuhlamba ukunyusa umphumo we-solubilizing we-surfactant kwioli.
②Ubushushu bunempembelelo ebaluleke kakhulu kwisenzo sokuphelisa ungcoliseko.Ngokubanzi, ukwandisa ubushushu kunceda ukususwa kokungcola, kodwa ngamanye amaxesha ubushushu obuphezulu kakhulu bunokubangela ukungalungi.
Ukunyuka kwamaqondo obushushu kuququzelela ukusasazwa kokungcola, igrisi eqinileyo i-emulsified ngokulula kumaqondo obushushu ngaphezu kwendawo yokunyibilika kwayo kwaye imicu yonyuka ekudumbeni ngenxa yokunyuka kwamaqondo obushushu, zonke ezo ziququzelela ukususwa kobumdaka.Nangona kunjalo, kwiimpahla ezidibeneyo, i-microgaps phakathi kweefayili ziyancitshiswa njengoko i-fibers ikhula, eyonakalisa ukukhutshwa kokungcola.
Utshintsho lobushushu luchaphazela ukunyibilika, ixabiso leCMC kunye nobungakanani bemicelle yee-surfactants, ngaloo ndlela kuchaphazela umphumo wokuhlamba.I-solubility ye-surfactants ene-carbon chain ende iphantsi kumaqondo aphantsi kwaye ngamanye amaxesha ukunyibilika kungaphantsi kwexabiso le-CMC, ngoko ke ubushushu bokuhlamba kufuneka buphakanyiswe ngokufanelekileyo.Isiphumo sobushushu kwixabiso leCMC kunye nobungakanani bemicelle buhlukile kwi-ionic kunye ne-non-ionic surfactants.Kwii-ionic surfactants, ukonyuka kobushushu kwandisa ixabiso le-CMC kwaye kunciphisa ubungakanani bemicelle, okuthetha ukuba ukuxinana kwe-surfactant kwisisombululo sokuhlamba kufuneka kwandiswe.Kwii-non-ionic surfactants, ukunyuka kweqondo lokushisa kukhokelela ekunciphiseni kwexabiso le-CMC kunye nokunyuka okukhulu kwevolumu ye-micelle, ngoko kuyacaca ukuba ukunyuka okufanelekileyo kwiqondo lokushisa kuya kunceda i-surfactant engeyiyo i-ionic ukuba isebenzise umphumo wayo osebenzayo. .Nangona kunjalo, iqondo lokushisa akufanele lidlule kwindawo yalo yelifu.
Ngamafutshane, ubushushu bokuhlamba obuphezulu buxhomekeke kwi-detergent kunye nento ehlanjwayo.Ezinye iidetergents zinesiphumo esihle sokucoca kwiqondo lobushushu begumbi, ngelixa ezinye zinesixhobo sokucoca esahluke kakhulu phakathi kokuhlamba okubandayo nokushushu.
③ Ugwebu
Kuyinto yesiko ukubhidanisa amandla ogwebu kunye nesiphumo sokuhlamba, ukholelwa ukuba ii-detergents ezinamandla amakhulu ogwebu zinefuthe elihle lokuhlamba.Uphando lubonise ukuba akukho budlelwane obuthe ngqo phakathi kwempembelelo yokuhlamba kunye nomthamo we-foam.Umzekelo, ukuhlamba ngezinto zokucoca ezinogwebu oluphantsi akusebenzi kangako kunokuhlamba ngezinto zokucoca ezinogwebu.
Nangona i-foam ayihambelani ngokuthe ngqo nokuhlamba, kukho amaxesha apho kunceda ukususa ukungcola, umzekelo, xa uhlamba izitya ngesandla.Xa ukhuhla iikhaphethi, i-foam inokuphinda isuse uthuli kunye namanye amasuntswana okungcola okuqinileyo, i-akhawunti yokungcola kwekhaphethi ngomlinganiselo omkhulu wothuli, ngoko ke ii-agent zokucoca ikhaphethi kufuneka zibe nobuchule obuthile bokukhupha amagwebu.
Amandla ogwebu nawo abalulekile kwiishampu, apho ugwebu olucolekileyo oluveliswa lulwelo ngexesha lokuhlanjwa kweshampu okanye ukuhlamba lushiya iinwele ziziva zithanjiswa kwaye zikhululekile.
④ Iintlobo ngeentlobo zeentsinga kunye neepropathi ezibonakalayo zelaphu
Ukongeza kwisakhiwo sekhemikhali semicu, echaphazela ukunamathela kunye nokususwa kokungcola, ukubonakala kweefayili kunye nokulungelelaniswa kwentambo kunye nelaphu kunempembelelo ekukhululekeni kokukhutshwa kokungcola.
Izikali zeentsinga zoboya kunye neerebhoni ezimcaba ezigobileyo zosinga lomqhaphu kunokwenzeka ukuba ziqokelele ubumdaka kunemisonto egudileyo.Ngokomzekelo, i-carbon black stained kwiifilimu ze-cellulose (iifilimu ze-viscose) zilula ukususa, ngelixa i-carbon black stained kwi-cotton fabrics kunzima ukuyihlamba.Omnye umzekelo ngowokuba amalaphu e-fiber emfutshane enziwe nge-polyester athanda ukuqokelela amabala e-oyile kunamalaphu e-fayibha ende, kwaye amabala e-oyile kumalaphu anefiber emfutshane anzima kakhulu ukuwasusa kunamabala e-oyile kwiilaphu ezinde.
Imisonto ephothiweyo kunye namalaphu aqinileyo, ngenxa yomsantsa omncinci phakathi kweentsinga, inokumelana nokuhlaselwa kokungcola, kodwa okufanayo kunokuthintela ulwelo lokuhlambela ukuba lungabandakanyi ubumdaka bangaphakathi, ke amalaphu aqinileyo aqala ukumelana nokungcola okulungileyo, kodwa xa sele engcolisiwe. ukuhlamba nako kunzima ngakumbi.
⑤ Ukuqina kwamanzi
Ukugxininiswa kwe-Ca2 +, i-Mg2 + kunye nezinye i-ion zetsimbi emanzini zinempembelelo enkulu kwimpembelelo yokuhlamba, ngakumbi xa i-anionic surfactants idibana ne-Ca2 + kunye ne-Mg2 + ion eyenza i-calcium kunye neetyuwa ze-magnesium ezinganyibilikiyo kwaye ziya kunciphisa ukucocwa kwayo.Kumanzi anzima, nokuba i-concentration ye-surfactant iphezulu, i-detergency isembi kakhulu kune-distillation.Ukuze i-surfactant ibe neyona nto ingcono yokuhlamba, ukuxinwa kwe-Ca2 + ion emanzini kufuneka kuncitshiswe kwi-1 x 10-6 mol / L (CaCO3 ukuya kwi-0.1 mg / L) okanye ngaphantsi.Oku kufuna ukongezwa kwezithambiso ezahlukeneyo kwisicoci.
Ixesha lokuposa: Feb-25-2022



